MEMS 2.0 enables the exchange of the Continuing Education data related to the FDA’s Risk Evaluation and Mitigation Strategy (REMS) for Opioids. Using MEMS 2.0, accreditors collect CE data from providers in a common format, allowing that data to be compiled across accreditation systems for reporting to the FDA. The data allows the FDA to get a sense of how many clinicians and which clinicians are participating in REMS CE. Data on 839 Opioid CE activities & 400,000+ learners has been reported to the FDA so far.
Using MEMS has many benefits:
- Ability to collect and analyze comprehensive CE outcomes data from multiple systems
- Easier collaboration with partners on the delivery and evaluation of CE
- Facilitates the development of standardized evaluation instruments
- Creates opportunity for industry wide data collection and research
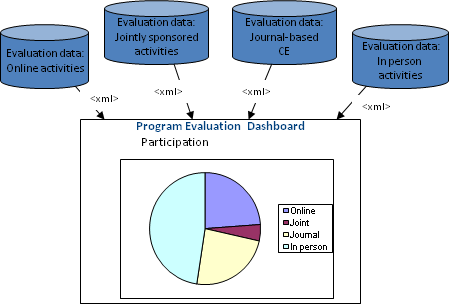
A System using MEMS to Bring Outcomes Data Together
MEMS provides a common format for the following types of data:
- Report description
- Activity description
- Participant survey data
- Knowledge assessment data
- Participation metrics, including credits awarded
MEMS was developed by the MedBiquitous Metrics Working Group. Visit the Metrics Working Group Page for member information.

